More Chemistry Facts
Atmospheric Chemistry:
- Knocking the NOx Out of Coal
- Ozone: Good Up High, Bad Nearby
- SO2: What is it? Where does it come from?
Chemical Composition:
Chemical Properties:
Chemical Reactions:
- Catalysts
- Chemical Burning
- Exploding Fertilizer
- Fire Retardant Gels
- How Sublime
- It's Crying Time Again
- Luminol; Trick-or-Treat or Terrible Feat
- Spontaneous Combustion
- Warmer Hands (And Toes) Through Chemistry
- What Are Aerosols?
- What Give Batteries Their Charge?
- What Is Reduction?
- What Makes a Candle Burn?
- When Chlorine Met Sodium...
- Why Does Cement Set?
- Why Doesn't Glue Get Hard In The Plastic Bottle?
Elements and Compounds:
- Hydrogen - The Simplest Element
- Hydrogen Reaction Experiment Reaps a Surprise
- Ice That Burns
- Nitrogen Gas and Compounds
- Radioactive Radon
- Radon, A Rare Element
- Table Salt - It's All In The Ions
- Turning Oil Into Gas
- Uses Of Hydrocarbons
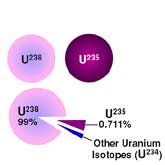
- What Are Isotopes?
- What Is A Half-life?
- What Is Acetone?
- What Is Arsenic?
- What Is The Periodic Table?