What Are Isotopes?
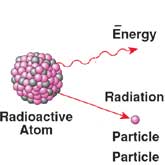 Many of the known elements from which our universe is constructed exist in various isotopic forms. The identity of any particular element is defined by the number of protons within the nuclei of its component atoms. All atoms with exactly six protons in their nuclei are thus identified as atoms of carbon, while all atoms with exactly ninety-two protons in their nuclei are defined to be atoms of uranium. Protons bear a positive charge, and since like charges repel each other, one might wonder how there can be more than one proton in a nucleus without that nucleus pushing itself apart as the protons try to get away from each other. Obviously, some type of 'nuclear glue' is required.
Many of the known elements from which our universe is constructed exist in various isotopic forms. The identity of any particular element is defined by the number of protons within the nuclei of its component atoms. All atoms with exactly six protons in their nuclei are thus identified as atoms of carbon, while all atoms with exactly ninety-two protons in their nuclei are defined to be atoms of uranium. Protons bear a positive charge, and since like charges repel each other, one might wonder how there can be more than one proton in a nucleus without that nucleus pushing itself apart as the protons try to get away from each other. Obviously, some type of 'nuclear glue' is required.
The role of nuclear glue is played by a subatomic particle known as the neutron. Neutrons are, as the name might suggest, electrically neutral. Their effectiveness in stabilizing polyprotonic nuclei is well evidenced by comparing the atomic structures of the two simplest elements, hydrogen and helium. The hydrogen atom consists of a single proton surrounded by a single electron. This is the most common element in the universe by far, and ostensibly the one from which all other elements in the universe have been made within the great nuclear fusion reactors called stars. Helium is the product of hydrogen fusion. Atoms of helium contain two protons and two neutrons, surrounded by two electrons. Rather than being destabilized by the presence of two protons confined within the space of the nucleus, helium atoms are so stabilized by the two neutrons that this element is the most stable, unreactive element known.
As the number of protons in the nucleus increases, so does the number of neutrons required to impart stability to the nucleus. Each type of atom requires a certain optimum number of neutrons to achieve this state. But as the number of neutrons increases, so too does the opportunity for some atoms to have either more or less than the optimum number of neutrons. Such atoms are known as isotopes, and the condition of not having the optimum number of neutrons in their nuclei allows them to spontaneously break apart into lighter, more stable atoms. Fortunately, they do not do this all at once, but at a rate that depends on the actual number of them that are present at the time.
About the Author
Richard M J Renneboog, MS
 Richard M. J. Renneboog is an independent private technical consultant and writer in both chemical and computer applications. Endeavors have included preparation of scripts for instructional and promotional video, corporate website design, curriculum development for training in advanced composites technology, and development.
Richard M. J. Renneboog is an independent private technical consultant and writer in both chemical and computer applications. Endeavors have included preparation of scripts for instructional and promotional video, corporate website design, curriculum development for training in advanced composites technology, and development.


