Water, Water Everywhere, But Not A Drop To Drink
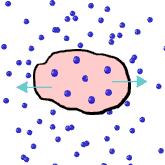 That line, from The Rime of the Ancient Mariner, by Samuel Taylor Coleridge, captures a truism -- we cannot drink salt water to quench our thirst. But why not? The answer lies in understanding the process of osmosis. Osmosis is the process whereby water molecules move from an area of higher concentration to an area of lower concentration. Osmosis occurs to stabilize a system. Think of putting ice cubes in a cup of hot chocolate. Besides being diluted with the water, the resulting liquid in the cup reaches an equilibrium between the two temperature extremes. This is similar to the way osmosis works. A higher concentration of water molecules will seek to reach an equilibrium with a lower concentration of water molecules.
That line, from The Rime of the Ancient Mariner, by Samuel Taylor Coleridge, captures a truism -- we cannot drink salt water to quench our thirst. But why not? The answer lies in understanding the process of osmosis. Osmosis is the process whereby water molecules move from an area of higher concentration to an area of lower concentration. Osmosis occurs to stabilize a system. Think of putting ice cubes in a cup of hot chocolate. Besides being diluted with the water, the resulting liquid in the cup reaches an equilibrium between the two temperature extremes. This is similar to the way osmosis works. A higher concentration of water molecules will seek to reach an equilibrium with a lower concentration of water molecules.
But what does that have to do with drinking salt water? Our cells are permeable membranes. That means some things can move in and out of cells through the cell wall, while other things cannot. Salt water is nothing more than water with suspended particles of natural salts. These salt particles are too large to pass through the cell walls. When taken into the bloodstream, salt water stays in the blood plasma and does not pass into the cells. In fact the reverse occurs.
If we were to drink salt water, and our blood plasma takes in the salt water, the salt in the water takes up space that the water molecules would normally take up. In effect, there is a lower concentration of water molecules in salt water. The water that is contained within the cells, is low in salt. That means there is a higher concentration of water molecules within the cells. So instead of water entering the cells to replenish them, water actually leaves the cells, dehydrating the cells even more. The more salt water you drink, the thirstier you become, until the major systems of your body start to shut down. Leave the salt water to the fish.
About the Author
Gene Mascoli, JD
 Gene Mascoli is a founder and publisher of ScienceIQ.com. He holds a J.D. degree from the University of Santa Clara and a B.A. in English. In 1997 Gene launched ScienceMaster.com, an online science education portal where he brought together his love of writing with his interest in the sciences. Gene collaborated with David Gamon on the popular digital book
“The Internet Guide to NASA on the Net” and has also produced two popular science CD-ROMs on astronomy and space science.
Gene Mascoli is a founder and publisher of ScienceIQ.com. He holds a J.D. degree from the University of Santa Clara and a B.A. in English. In 1997 Gene launched ScienceMaster.com, an online science education portal where he brought together his love of writing with his interest in the sciences. Gene collaborated with David Gamon on the popular digital book
“The Internet Guide to NASA on the Net” and has also produced two popular science CD-ROMs on astronomy and space science.


