Neutrinos to the Rescue
 Have you ever wondered what the most abundant particle in the universe is after photons of light? The answer is: Neutrinos. These tiny, neutral and almost mass-less particles that move at almost the speed of light hardly ever interact with anything in the universe. In fact about ten thousand trillion neutrinos will pass through your body by the time you are finished reading this.
Have you ever wondered what the most abundant particle in the universe is after photons of light? The answer is: Neutrinos. These tiny, neutral and almost mass-less particles that move at almost the speed of light hardly ever interact with anything in the universe. In fact about ten thousand trillion neutrinos will pass through your body by the time you are finished reading this.
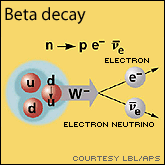
The existence of neutrinos was predicted by Wolfgang Pauli in 1930. After observing the beta decay, a process where a neutron (which was not yet discovered at the time) from atom's nucleus decays into a proton and an electron, it was noticed that the energy just did not add up. Namely, there was a missing amount of energy that was a threat to the well-established law of conservation of energy. Pauli then postulated that there must be a new particle which was not seen that would carry this missing difference in energy. He named it the 'neutron'. This name did not last too long since in 1932 James Chadwick actually discovered the neutron. Fermi then renamed it a neutrino, which in Italian means: little neutral one. It was only in 1956 that Clyde Cowan and Fredrick Reines actually detected neutrinos from a nuclear power plant for the first time.
Most of the neutrinos in the universe were created during the first few seconds after the Big Bang. Thanks to their weak interaction with matter, most of those neutrinos are still around. Neutrinos are also created in nuclear power plants and in our Sun and other stars where, in the process of fusion, four protons and two electrons get fused into an atom of Helium and in the process create two neutrinos. We still know very little about these elusive particles, namely that their mass is very small (smaller than that of the electron), but we don't know exactly what that mass is. We also believe that they travel at or close to the speed of light, but again we are not sure what that speed is. Further research into neutrinos will not only answer these questions but will also allow us to peek into the early universe, to learn about the formations of stars and explosions of supernovas. The message is in the neutrinos.